0898-08980898 13876453617
1.就根据标准计划提交的申请,申请人应是持有相关牌照,例如批发商牌照、抗生素许可证及危险药物批发商牌照(视何者适用)的本地公司,或进行有关试验的首席研究者。
2.就根据列载计划提交的申请,只有发起并进行试验的申办者-研究者才应作为申请人。
1.填妥的申请表;
2. 计划书;
3. 病人同意书样本;
4. 道德委员会的批准文件;
5. 研究者手册
6. 首席研究者的意向书和履历;
7. 相关的药品生产质量管理规范证明书
8. 化验分析证明书;以及
9. 其他相关文件(如有的话)
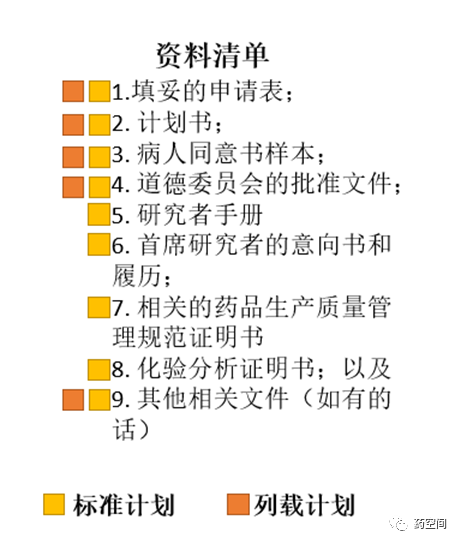
伦理递交与监管机构递交可平行进行,伦理批准文件可稍晚递交给监管机构。
伦理批准大概需要12周(非官方信息)
申請人應於證明書屆滿前不少於 4 個月提交新申請。(来源官方信息)
预估政府4-6周批准,外加1周申请药物进口许可证。总的批准时间大概为9-16周。(非官方信息)
未查询到Pre-IND要求
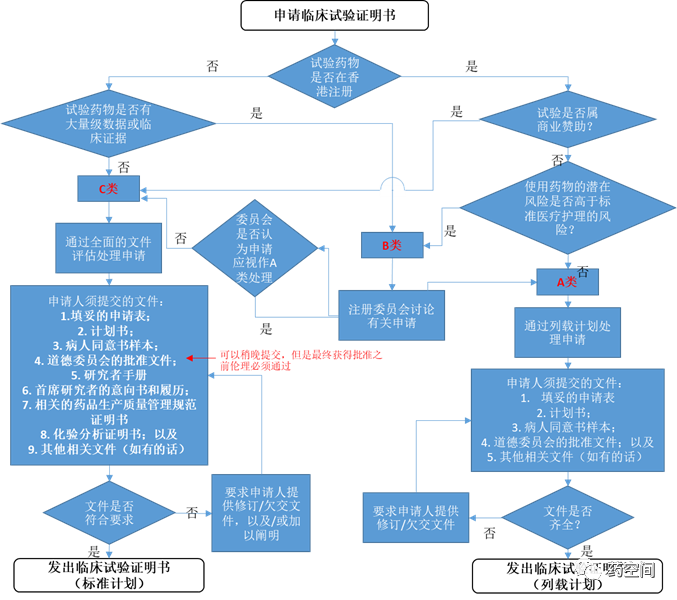
《临床试验/药物测试证明书》参考链接:http://www.drugoffice.gov.hk/gb/unigb/www.drugoffice.gov.hk/eps/do/tc/pharmaceutical_trade/guidelines_forms/clinicalTrialCertAppl.html
药物进出口申请及相关牌照申请指南参考链接:
To obtain approval of a clinical trial protocol in South Korea, a foreign company without an established presence in Korea must delegate allrights and responsibilities for the execution of the clinical trial through anagreement with a contract research organization (CRO) established in Korea
1. IND application
2. Development Plan
3. Investigator’s Brochure (IB)
4. Documentation or data that prove theinvestigational drug is manufactured in compliance with Appendix 1, 4-2
5. Data on drug substance and its quantity,manufacturing method, and manufacturer of the investigational drug
6. Data of nonclinical test results:Toxicity, pharmacological, and ADME
7. Data on the prior clinical use of theinvestigational product (if available)
8. Data on clinical trial institution,investigator, and contract research institute according to Article 34-2 (2) ofthe Act
9. Rules on compensation for victims ofclinical trial
10. Trial subject’s informed consent form
11. Clinical trial protocol
伦理递交与CTA监管机构递交可平行进行,审核时间约50天。
The process for regulatory approval takes about 30 days.
1. Ensuring quality standards, etc. Companiesshould use the MFDS consulting services for pre-investigational new drugs(Pre-IND) to determine which documents will be needed.
2. An import permit will be required beforethe investigational product is shipped into Korea.
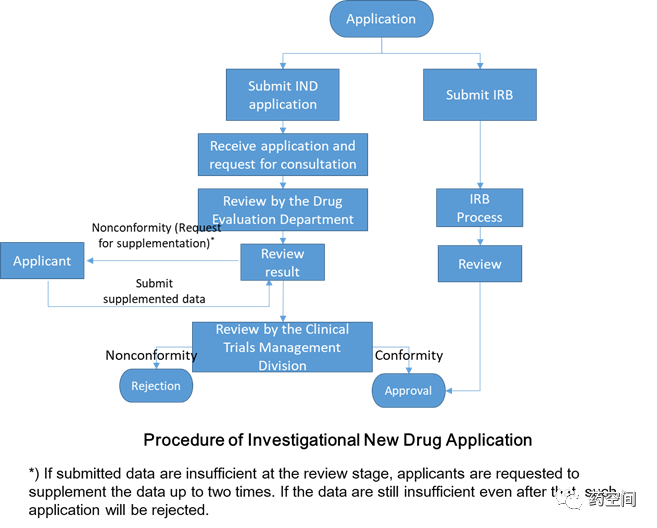
《Guide to DrugApproval System in Korea》:https://www.kobia.kr/skin/bbs/downloads_e2/download.php?tbl=policy_report&no=407
There is no requirement that the ultimate sponsor of a clinicaltrial must be located in Singapore and/or the region around Singapore. However,applications for a CTA, CTN or CTC are to be made by a local representative (inits capacity as a local sponsor), which must be a locally registered company.
1. Clinical trial protocol
2. Informed consent form (in English)
3. Investigator's brochure for locallyunregistered products
4. Approved product label for locallyregistered products
5. List of overseas trial sites (whereapplicable)
6. Principal investigator's CV
7. Good Manufacturing Practice (GMP)certificate
8. Certificate of Analysis (COA) for studybatches of investigational products
9. Chemistry, Manufacturing and Control (CMC)information, when requested
10. Documents for CRM Notification, ifapplicable:
10.1 List of components in a medical devicesystem
10.2 Packing list for study-visits specific labkits, if products in lab kits are not declared in application form
伦理递交与CTA监管机构递交可平行进行
CTA:30 working days;
未查询到相关Pre-IND要求,但是在完成申请草案后,当地申办者代表将通知相关方(例如,主要研究者、其他申办者、CRM 进口商或本地制造商,如适用)以认可该申请。 一旦所有相关方完成授权,当地申办方才可继续递交申请。
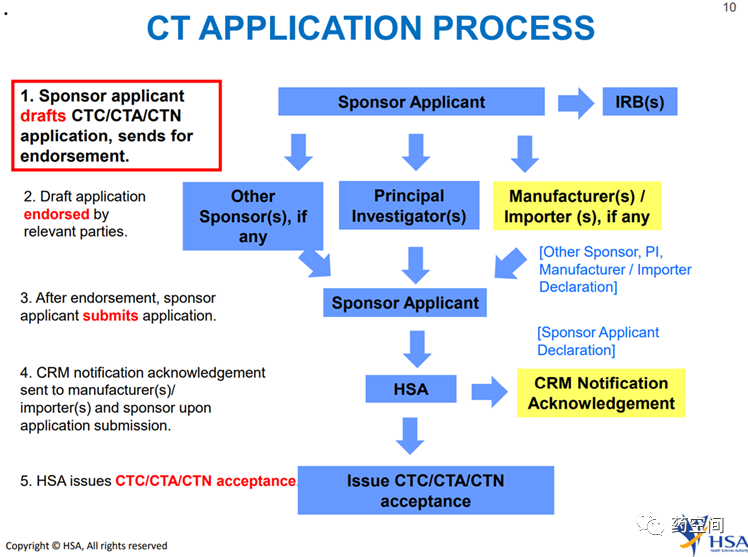
https://www.hsa.gov.sg/clinical-trials/application/apply-cta-ctn
According to ECMOPH which is one of the ethics committees approvedby th Thai FDA, to approve clinical research protocols, requires that the sponsor and/or CRO be legally registered in Thailand.
1. Research Proposal
2. Proposal for ethical consideration signedby the investigator/co-investigator (or advisor)
3. Research protocol transcript (in Thaiand/or the complete English version specifying the version and date); or theThai version if only the complete English outline of the proposal is included
4. Documents detailing the joint researchproject data/books consent (the Information sheet/Consent Form) (if required)(page number/version and date; noting in the event a participant has children;including documents specifying if participants are children and/or if assentform is required)
5. Investigator/co-investigator Curriculumvitaes (CVs)
6. Evidence of good clinical practice (GCP)training or research ethics training by PIs/joint investigators
7. Conflict of Interest Form completed byPIs/joint investigators
8. CD containing one (1) copy of researchproject electronic file
伦理递交与CTA监管机构递交可平行进行(时间约2-3个月,信息来源非官方)
Thai FDA
Thai FDA’s review and approval process fordrugs to be imported for clinical trials takes 20 working days upon receipt ofthe application
In addition, once the Thai FDA receives theEC approval documentation, the agency will complete its review within 15 days
EC
The review and approval process by a ThaiFDA recognized EC will vary by institution (2-3 months).
1. 未查询到相关Pre-IND要求。
2. 泰国FDA通过授予研究药物进口许可来间接管理临床试验,而泰国决定允许研究药物进口取决于伦理委员会对临床试验的批准。
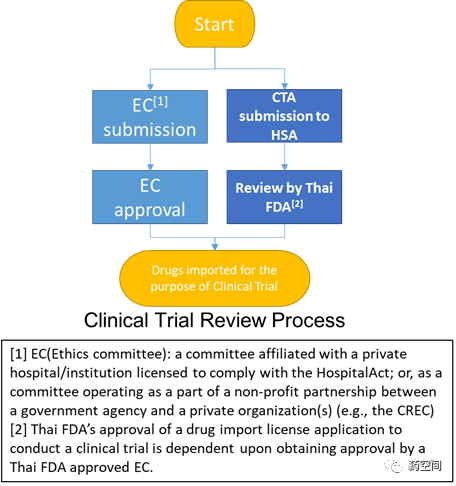
https://clinregs.niaid.nih.gov/country/thailand#submission_process
1. The sponsor is defined as an individual, entity, or organizationthat takes responsibility for the initiation, management, and/or financing of aclinical trial.
2. In addition, per PharmLaw, the sponsor mayselect a qualified contract research organization (CRO) (also known as aresearch support organization in Vietnam) to run the clinical trial.
3. According to PharmLaw, a sponsor andhis/her CRO may be domestic or foreign.
监管机构以及伦理委员会需要的材料清单可以在以下页面查看:https://clinregs.niaid.nih.gov/country/vietnam#submission_content
1. 伦理递交与CTA监管机构递交可同时进行。
2. 申请人递交文件给监管机构MOH后,MOH会组织国家生物医学研究伦理委员会(NECBR)审阅Research Proposal。但是在获得NECBR批准前需先获得试验机构伦理委员会的批准。
MOH和NECBR审阅时间约为40天。(详见流程图)
机构伦理委员会审阅时间约1周。
未查询到相关Pre-IND要求
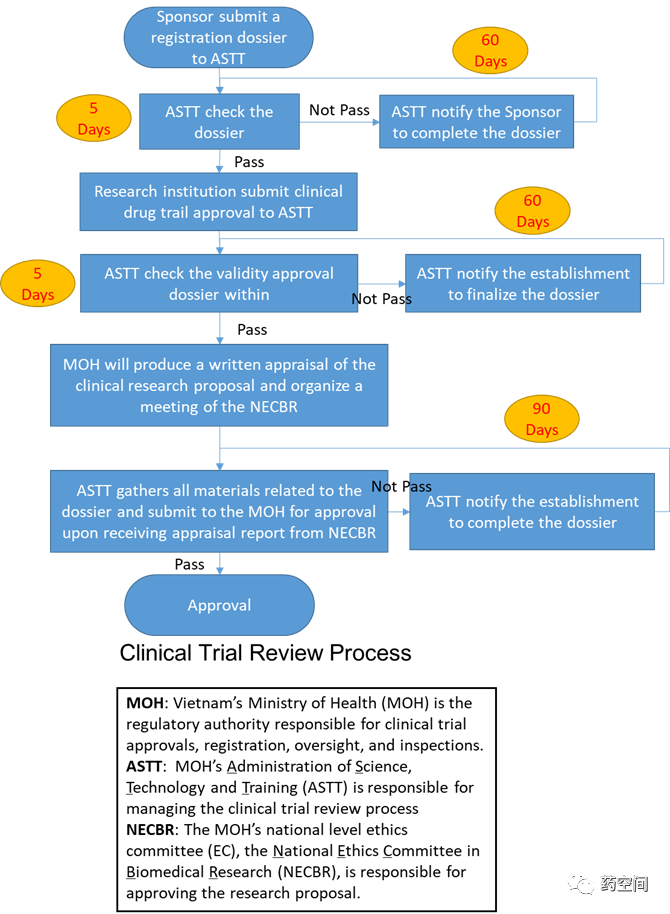
https://clinregs.niaid.nih.gov/country/vietnam#regulatory_authority
The sponsor is not required to be located in Japan. However, if a person who intends to sponsor a clinical trial is not located in Japan, the sponsor or prospective sponsor will need to appoint an in-country clinical caretaker who has residency in Japan(including the representative of a foreign legal person holding office in Japan) so that person can carry out the necessary procedures for sponsoring a clinical trial and ensure that necessary measures are taken to prevent the occurrence or spread of hazards to public health and hygiene
1. Statement regarding the reason why the sponsoring of the proposedclinical trial is scientifically justified.
2. A protocol of the proposed clinical trial
3. An explanation document used for informed consent and consent form
4. Sample of Case Report Form
5. Current investigator's brochure
Once the PMDA completes its review, then the next step is to getapproval from the Institutional Review Board (IRB). The IRB takes about 1-4 weeks for review and approval.
It may probably take 30 days for the initial INDconsultations but later may take 14 days for second and consecutiveINDs.
Pre-IND is recommended to ensure a flawless and streamlinedIND application process but not mandatory.
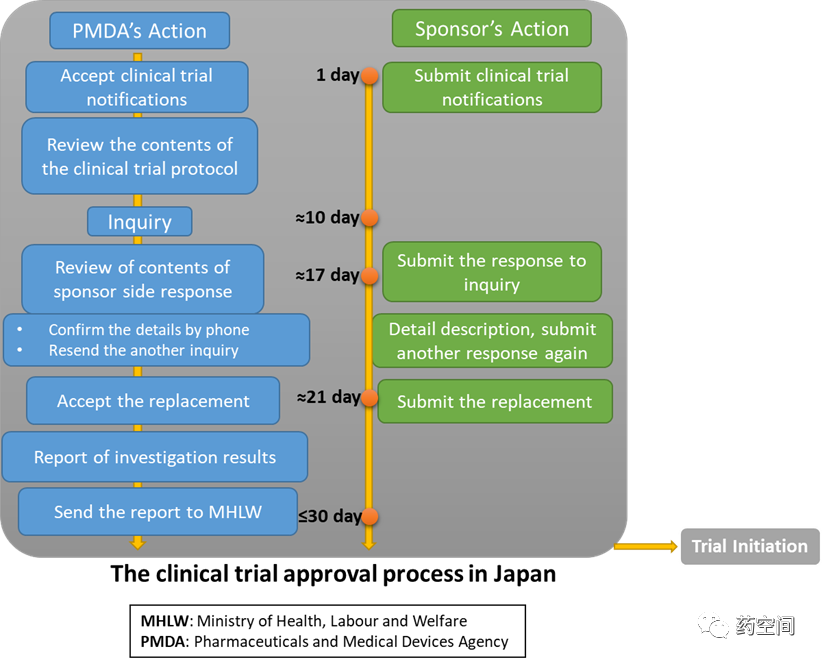
https://www.pmda.go.jp/files/000152326.pdf
https://credevo.com/articles/2020/04/15/the-drug-approval-process-in-japan/
提醒:以上讯息由作者Senatus通过网络搜集整理,仅供参考。详细的法规条例以各国官方监管机构发布信息为准。
如果对您有帮助,记得转载、收藏、点赞!
记得关注”药空间“获取更多医药相关资讯哦~!